SCIENCE
Your promising partner CellabMED
YYB-101
| Project | Target | Indication | Development phase |
|---|---|---|---|
| YYB-101 | HGF | Colorectal cancer | Phase 2a |
Summary
YYB-101 is a neutralizing antibody targeting hepatocyte growth factor (HGF), and a new anticancer antibody drug blocking HGF/c-Met signaling pathway. HGF and cMET have been reported to be overexpressed in cancerous tissues including the liver, prostate, colon, breast, brain and skin, and it is known that such overexpression is highly correlated with the prognosis and possibility of metastasis in cancer patients. YYB-101 has demonstrated its excellent efficacy in animal experiments on brain cancer, sarcoma, ovarian cancer, colorectal cancer, etc., and has been confirmed to be a very safe drug in phase 1 clinical trials in patients with solid tumors who have not responded to standard therapies. Phase 2a clinical trials have been completed in patients with metastatic colorectal cancer, and we plan to expand the treatment's indications to melanoma and pancreatic cancer.
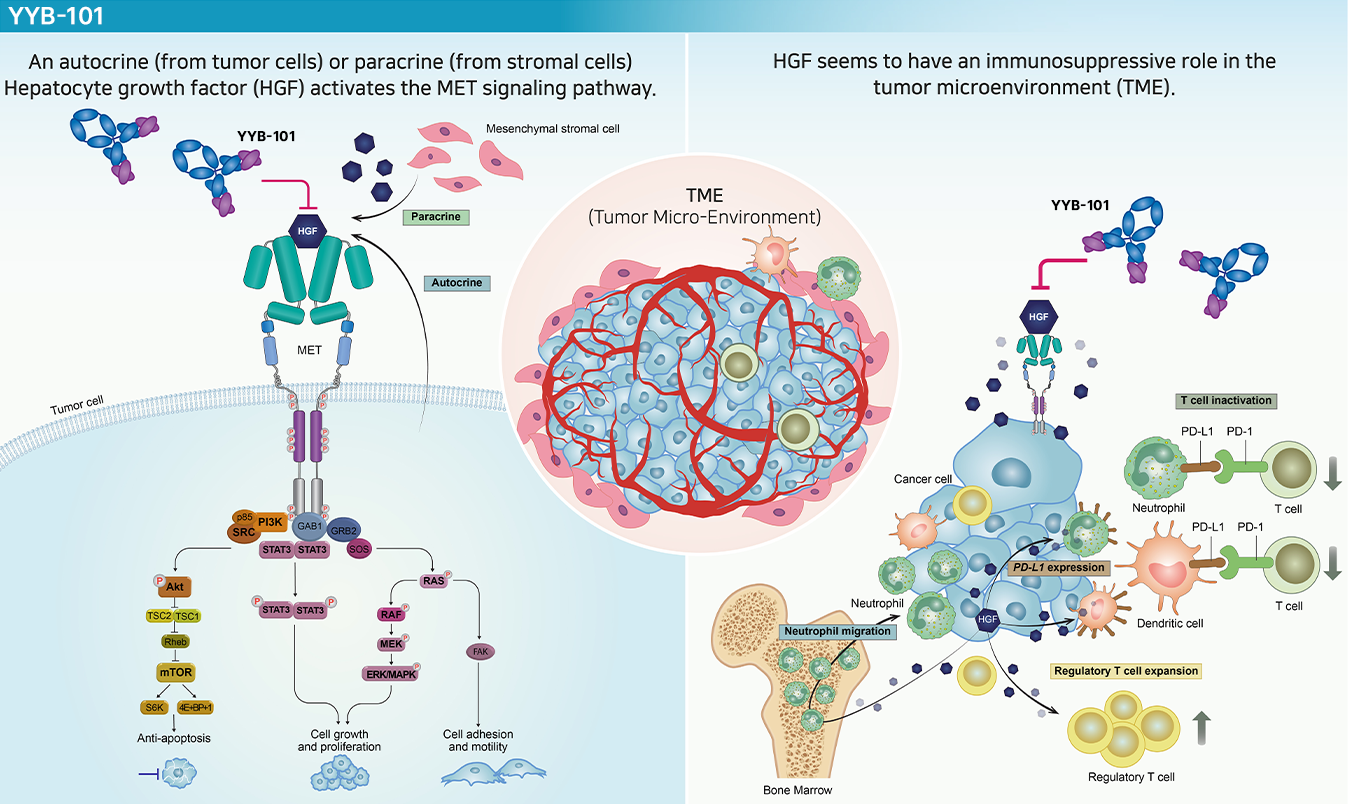
Structure and mechanism of action
YYB-101 is a humanized monoclonal antibody targeting hepatocyte growth factor (HGF). It has an anticancer mechanism that interferes with binding to the receptor c-Met, blocking the MET signaling pathway and inhibiting activation. It also inhibits tumor growth, scattering, motility, invasiveness and metastasis.
Technical advantage
- YYB-101 is a highly safe IgG4-based monoclonal antibody that eliminates non-specific ADCC/CDC functions.
- With YYB-101's much higher antibody affinity thanother available products, excellent anticancer therapeutic effect is anticipated.
- YYB-101 completely inhibits binding between HGF and the receptor c-Met due to its unique epitope.
- YYB-101’s excellent safety profiles in phase 1 and phase 2 clinical trials confirm its safety efficacy.
Patent
| Project | Application/Registration No. | Status | Name of invention | Application date | Domestic/Foreign | Right holder |
|---|---|---|---|---|---|---|
| *Technical transfer | ||||||
| YYB-101 | KR 10-0556660 | Registration | Neutralizing epitope of HGF and neutralizing antibody binding to HGF | 2003.11.11 | South Korea | CellabMED |
| YYB-101 | US 7408043 | Registration | Neutralizing antibody against HGF | 2006.05.10 | US | CellabMED |
| YYB-101 | ZL 200480033164.9 | Registration | 可中和的HGF表位和与其结合的中和抗体 | 2006.05.11 | China | CellabMED |
| YYB-101 | JP 4446393 | Registration | Hgfの中和可能エピトープ及びこれに結合する中和抗体 | 2006.05.11 | Japan | CellabMED |
| YYB-101 | EP 1 694 700 | Registration | NEUTRALIZABLE EPITHOPE OF HGF AND NEUTRALIZING ANTIBODY THAT JOINS THE SAME | 2006.06.08 | Europe | CellabMED |
| YYB-101 | IN 249026 | Registration | Neutralizable epitope of hgf and neutralizing antibody binding to the same | 2006.06.12 | India | CellabMED |
| YYB-101 | HK 1098769 | Registration | Neutralizable epitope of hgf and neutralizing antibody binding to the same | 2006.06.13 | Hong Kong | CellabMED |
| YYB-101 | AU 2004287743 | Registration | Neutralizable epitope of HGF and neutralizing antibody binding to the same | 2009.01.22 | Australia | CellabMED |
| YYB-101 | KR 10-0829972 | Registration | Anti-HGF/SF humanized antibody and preparation method | 2006.07.14 | South Korea | CellabMED |
| YYB-101 | JP 4509069 | Registration | Anti-HGF / SF humanized antibody and method for producing the same | 2006.07.14 | Japan | CellabMED |
| YYB-101 | US 7718174 | Registration | Anti-HGF/SF humanized antibody | 2007.01.13 | US | CellabMED |